Abstract
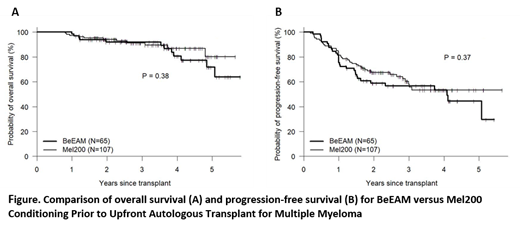
Single-agent high-dose melphalan followed by autologous stem cell transplantation (ASCT) remains a standard of care in eligible patients with multiple myeloma (MM). Efforts to improve transplant outcomes by intensifying transplant conditioning have mostly failed, with the exception of a sole phase III study showing improved progression-free survival (PFS) with the addition of busulfan to melphalan (Lancet Haematology 2019, 6:266-75). Bendamustine is a synthetic agent that combines the alkylating properties of a mustard-group with the antimetabolic activity of a purine analog. It can induce responses in MM resistant to other alkylators and is therefore a promising agent to test synergy in conditioning regimens. The high-dose chemotherapy combination of bendamustine, etoposide, cytarabine and melphalan (BeEAM) has been shown to be a safe and effective transplant regimen for lymphoma patients (Blood 2011, 118:3419-25). We performed a phase II study to test the safety and efficacy of BeEAM in MM patients, 18-70 years of age, with adequate organ function (EF≥40% predicted, CrCl ≥40ml/min) and within 9 months of starting induction therapy. Results were compared to a contemporaneously treated control group receiving a single high-dose melphalan 200mg/m2 transplant (Mel200) but otherwise meeting study eligibility criteria. Study patients (n=65) had a median (range) age of 59 (40, 69) years and were transplanted from 2015-2020. Other characteristics included KPS<80%, HCT-CI≥3, ISS III, and high-risk FISH in 35%, 46%, 26% and 44% respectively. Pre-BMT status was (s)CR1, VGPR1, PR1 and <PR1 in 26%, 43%, 26% and 5% respectively. ASCT following BeEAM was well tolerated with no non-relapse deaths through one-year post-transplant. Although at least one non-hematologic grade 3 toxicity was reported in 32 (49%) patients, there were no grade ≥3 renal or hepatic toxicity and no grade ≥4 non-hematologic toxicity. With a median f/u of 44 (13, 70) months, three-year OS and PFS was 92% and 57% respectively. When BeEAM patients were compared to contemporaneously treated Mel200 patients, there were no significant differences in baseline characteristics, induction regimen or use of post-transplant maintenance therapy. Neutrophil and platelet engraftment was faster following BeEAM (11 vs. 12 days, 17 vs 18 days, p<0.001 and p=0.007 respectively), when compared to Mel200 patients. One-year non-relapse mortality was 0% with both BeEAM and Mel200. No significant differences were seen in quality of response, PFS or OS between BeEAM and Mel200, when analyzing either the whole cohort (see figure) or by genetic risk stratification. In multivariate analysis, controlled for genetic risk and year of transplantation, the use of BeEAM conditioning offered no benefit to Mel200 in terms of OS, PFS or risk of relapse/progression. In summary, BeEAM was shown to be a safe and effective conditioning regimen prior to up-front autologous transplant for MM. However, BeEAM conditioning appears to offer no significant advantage when compared to conventional Mel200 conditioning.
Solh: Jazz Pharmaceuticals: Consultancy; BMS: Consultancy; Partner Therapeutics: Research Funding; ADCT Therapeutics: Consultancy, Research Funding.
Bendamustine for Multiple Myeloma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal